Active transport
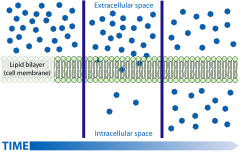
Active transport is the movement of molecules across a cell membrane from a region of their lower concentration to a region of their higher concentration—in the direction against some gradient or other obstructing factor (often a concentration gradient).
Unlike passive transport, which uses the kinetic energy and natural entropy of molecules moving down a gradient, active transport uses cellular energy to move them against a gradient, polar repulsion, or other resistance. Active transport is usually associated with accumulating high concentrations of molecules that the cell needs, such as ions, glucose and amino acids. If the process uses chemical energy, such as from adenosine triphosphate (ATP), it is termed primary active transport. Secondary active transport involves the use of an electrochemical gradient. Examples of active transport include the uptake of glucose in the intestines in humans and the uptake of mineral ions into root hair cells of plants.
History
In 1848, the German physiologist Emil Heinrich du Bois-Reymond suggested the possibility of active transport of substances across membranes.
Rosenberg (1948) formulated the concept of active transport based on energetic considerations, but later it would be redefined.
Details
Specialized transmembrane proteins recognize the substance and allow it to move across the membrane when it otherwise would not, either because the phospholipid bilayer of the membrane is impermeable to the substance moved or because the substance is moved against the direction of its concentration gradient. There are two forms of active transport, primary active transport and secondary active transport. In primary active transport, the proteins involved are pumps that normally use the chemical energy in the form of ATP. Secondary active transport, however, makes use of potential energy, which are usually derived through exploitation of an electrochemical gradient. This involves pore-forming proteins that form channels across the cell membrane. The difference between passive transport and active transport is active transport requires energy and moves substances against their respective concentration gradient, whereas passive transport requires no energy and moves substances in the direction of their respective concentration gradient.
In an antiporter, one substrate is transported in one direction across the membrane while another is cotransported in the opposite direction. In a symporter, two substrates are transported in the same direction across the membrane. Antiport and symport processes are associated with secondary active transport, meaning that one of the two substances is transported in the direction of its concentration gradient, utilizing the energy derived from the transport of such substance (mostly Na+, K+ or H+ ions) down its concentration gradient.
If substrate molecules are moving from areas of lower concentration to areas of higher concentration (i.e., in the opposite direction as, or against the concentration gradient), specific transmembrane carrier proteins are required. These proteins have receptors that bind to specific molecules (e.g., glucose) and transport them across the cell membrane. Because energy is required in this process, it is known as 'active' transport. Examples of active transport include the transportation of sodium out of the cell and potassium into the cell by the sodium-potassium pump. Active transport often takes place in the internal lining of the small intestine.
Plants need to absorb mineral salts from the soil or other sources, but these salts exist in very dilute solution. Active transport enables these cells to take up salts from this dilute solution against the direction of the concentration gradient.
Primary active transport
Primary active transport, also called direct active transport, directly uses metabolic energy to transport molecules across a membrane.
Most of the enzymes that perform this type of transport are transmembrane ATPases. A primary ATPase universal to all animal life is the sodium-potassium pump, which helps to maintain the cell potential. The sodium-potassium pump maintains the membrane potential by moving three Na+ ions out of the cell for every two K+ ions moved into the cell. Other sources of energy for Primary active transport are redox energy and photon energy (light). An example of primary active transport using Redox energy is the mitochondrial electron transport chain that uses the reduction energy of NADH to move protons across the inner mitochondrial membrane against their concentration gradient. An example of primary active transport using light energy are the proteins involved in photosynthesis that use the energy of photons to create a proton gradient across the thylakoid membrane and also to create reduction power in the form of NADPH.
Model of active transport
ATP hydrolysis is used to transport hydrogen ions against the electrochemical gradient (from low to high hydrogen ion concentration). Phosphorylation of the carrier protein and the binding of a hydrogen ion induce a conformational (shape) change that drives the hydrogen ions to transport against the electrochemical gradient. Hydrolysis of the bound phosphate group and release of hydrogen ion then restores the carrier to its original conformation.
Types of ATP-powered primary active transporters
- P-type ATPase: sodium potassium pump, calcium pump, proton pump
- F-ATPase: mitochondrial ATP synthase, chloroplast ATP synthase
- V-ATPase: vacuolar ATPase
- ABC (ATP binding cassette) transporter: MDR, CFTR, etc.
Secondary active transport
In secondary active transport, also known as coupled transport or co-transport, energy is used to transport molecules across a membrane; however, in contrast to primary active transport, there is no direct coupling of ATP; instead it relies upon the electrochemical potential difference created by pumping ions in/out of the cell. Permitting one ion or molecule to move down an electrochemical gradient, but possibly against the concentration gradient where it is more concentrated to that where it is less concentrated increases entropy and can serve as a source of energy for metabolism (e.g. in ATP synthase).
In August 1960, in Prague, Robert K. Crane presented for the first time his discovery of the sodium-glucose cotransport as the mechanism for intestinal glucose absorption. Crane's discovery of cotransport was the first ever proposal of flux coupling in biology.
Cotransporters can be classified as symporters and antiporters depending on whether the substances move in the same or opposite directions.
Antiport
In an antiport two species of ion or other solutes are pumped in opposite directions across a membrane. One of these species is allowed to flow from high to low concentration which yields the entropic energy to drive the transport of the other solute from a low concentration region to a high one.
An example is the sodium-calcium exchanger or antiporter, which allows three sodium ions into the cell to transport one calcium out.Many cells also possess calcium ATPases, which can operate at lower intracellular concentrations of calcium and sets the normal or resting concentration of this important second messenger. But the ATPase exports calcium ions more slowly: only 30 per second versus 2000 per second by the exchanger. The exchanger comes into service when the calcium concentration rises steeply or "spikes" and enables rapid recovery. This shows that a single type of ion can be transported by several enzymes, which need not be active all the time (constitutively), but may exist to meet specific, intermittent needs.
Symport
Symport uses the downhill movement of one solute species from high to low concentration to move another molecule uphill from low concentration to high concentration (against its concentration gradient). Both molecules are transported in the same direction.
An example is the glucose symporter SGLT1, which co-transports one glucose (or galactose) molecule into the cell for every two sodium ions it imports into the cell. This symporter is located in the small intestines, heart, and brain. It is also located in the S3 segment of the proximal tubule in each nephron in the kidneys. Its mechanism is exploited in glucose rehydration therapy and defects in SGLT1 prevent effective reabsorption of glucose, causing familial renal glucosuria.
Examples
- Metal ions, such as Na+, K+, Mg2+, or Ca2+, require ion pumps or ion channels to cross membranes and distribute through the body
- The pump for sodium and potassium is called sodium-potassium pump or Na +/K+-ATPase
- In the epithelial cells of the stomach, gastric acid is produced by hydrogen potassium ATPase, an electroneutral pump
- Water, ethanol, and chloroform exemplify simple molecules that do NOT require active transport to cross a membrane.
Endocytosis and Exocytosis
Endocytosis and exocytosis are both forms of bulk transport that move materials into and out of cells, respectively, via vesicles. In the case of Endocytosis, the cellular membrane folds around the desired materials outside the cell. The ingested particle becomes trapped within a pouch, known as a vesicle, inside the cytoplasm. Often enzymes from lysosomes are then used to digest the molecules absorbed by this process.
Biologists distinguish two main types of endocytosis: pinocytosis and phagocytosis.
- In pinocytosis, cells engulf liquid particles (in humans this process occurs in the small intestine, where cells engulf fat droplets).
- In phagocytosis, cells engulf solid particles.
Passive transport
Passive transport is a movement of ions and other atomic or molecular substances across cell membranes without need of energy input. Unlike active transport, it does not require an input of cellular energy because it is instead driven by the tendency of the system to grow in entropy. The rate of passive transport depends on the permeability of the cell membrane, which, in turn, depends on the organization and characteristics of the membrane lipids and proteins. The four main kinds of passive transport are simple diffusion, facilitated diffusion, filtration, and osmosis.Diffusion
Diffusion is the net movement of material from an area of high concentration to an area with lower concentration. The difference of concentration between the two areas is often termed as the concentration gradient, and diffusion will continue until this gradient has been eliminated. Since diffusion moves materials from an area of higher concentration to an area of lower concentration, it is described as moving solutes "down the concentration gradient" (compared with active transport, which often moves material from area of low concentration to area of higher concentration, and therefore referred to as moving the material "against the concentration gradient"). However, in many cases (e.g. passive drug transport) the driving force of passive transport can not be simplified to the concentration gradient. If there are different solutions at the two sides of the membrane with different equilibrium solubility of the drug, the difference in degree of saturation is the driving force of passive membrane transport. It is also true for supersaturated solutions which are more and more important owing to the spreading of the application of amorphous solid dispersions for drug bioavailability enhancement.Simple diffusion and osmosis are in some ways similar. Simple diffusion is the passive movement of solute from a high concentration to a lower concentration until the concentration of the solute is uniform throughout and reaches equilibrium. Osmosis is much like simple diffusion but it specifically describes the movement of water (not the solute) across a selectively permeable membrane until there is an equal concentration of water and solute on both sides of the membrane. Simple diffusion and osmosis are both forms of passive transport and require none of the cell's ATP energy.Facilitated diffusion
Facilitated diffusion, also called carrier-mediated osmosis, is the movement of molecules across the cell membrane via special transport proteins that are embedded within the cellular membrane. Large, insoluble molecules, such as glucose, vesicles and proteins require a carrier molecule to move through the plasma membrane. Therefore, it will bind with its specific carrier proteins, and the complex will then be bonded to a receptor site and moved through the cellular membrane. Facilitated diffusion is a passive process: the solutes move down their concentration gradient and do not require the expenditure of cellular energy for this process. Carrier proteins and channel proteins allow for the diffusion of molecules across the cell membrane. Carrier proteins undergo conformational alterations to allow molecules to pass, while channel proteins form unblocked pores.Facilitated diffusion may be achieved as a consequence of charge gradients in addition to concentration gradients. Plant cells create an unequal distribution of charge across their plasma membrane by actively taking up or excluding ions. Active transport of protons by H+ ATPases alters membrane potential allowing for facilitated passive transport of particular ions such as Potassium down their charge gradient through high affinity transporters and channels.Filtration
Filtration is movement of water and solute molecules across the cell membrane due to hydrostatic pressure generated by the cardiovascular system. Depending on the size of the membrane pores, only solutes of a certain size may pass through it. For example, the membrane pores of the Bowman's capsule in the kidneys are very small, and only albumins, the smallest of the proteins, have any chance of being filtered through. On the other hand, the membrane pores of liver cells are extremely large,but not forgetting cells are extremely small to allow a variety of solutes to pass through and be metabolized.Osmosis
Osmosis is the movement of water molecules across a selectively permeable membrane. The net movement of water molecules through a partially permeable membrane from a solution of high water potential to an area of low water potential. A cell with a less negative water potential will draw in water but this depends on other factors as well such as solute potential (pressure in the cell e.g. solute molecules) and pressure potential (external pressure e.g. cell wall). There are three types of Osmosis solutions: the isotonic solution, hypotonic solution, and hypertonic solution. Isotonic solution is when the extracellular solute concentration is balanced with the concentration inside the cell. In the Isotonic solution, the water molecules still moves between the solutions, but the rates are the same from both directions, thus the water movement is balanced between the inside of the cell as well as the outside of the cell. A hypotonic solution is when the solute concentration outside the cell is lower than the concentration inside the cell. In hypotonic solutions, the water moves into the cell, down its concentration gradient (from higher to lower water concentrations). That can cause the cell to swell. Cells that don't have a cell wall, such as animal cells, could burst in this solution. A hypertonic solution is when the solute concentration is higher (think of hyper - as high) than the concentration inside the cell. In hypertonic solution, the water will move out, causing the cell to shrink.-
- References
- 1. wikipedia the free encyclopedia.
- 2. Borbas, E.; et al. (2016). "Investigation and Mathematical Description of the Real Driving Force of Passive Transport of Drug Molecules from Supersaturated Solutions". Molecular Pharmaceutics. 13: 3816–3826. doi:10.1021/acs.molpharmaceut.6b00613









No comments:
Post a Comment