Chloroplast
Chloroplasts /ˈklɔːrəˌplæsts, -plɑːsts/ are organelles, specialized subunits, in plant and algal cells. Their discovery inside plant cells is usually credited to Julius von Sachs (1832–1897), an influential botanist and author of standard botanical textbooks – sometimes called "The Father of Plant Physiology".
The main role of chloroplasts is to conduct photosynthesis, where the photosynthetic pigment chlorophyll captures the energy from sunlight and converts it and stores it in the energy-storage molecules ATP and NADPH while freeing oxygen from water. They then use the ATP and NADPH to make organic molecules from carbon dioxide in a process known as the Calvin cycle. Chloroplasts carry out a number of other functions, including fatty acid synthesis, much amino acid synthesis, and the immune response in plants. The number of chloroplasts per cell varies from one, in unicellular algae, up to 100 in plants like Arabidopsis and wheat.
A chloroplast is a type of organelle known as a plastid, characterized by its high concentration of chlorophyll. Other plastid types, such as the leucoplast and the chromoplast, contain little chlorophyll and do not carry out photosynthesis.
Chloroplasts are highly dynamic—they circulate and are moved around within plant cells, and occasionally pinch in two to reproduce. Their behavior is strongly influenced by environmental factors like light color and intensity. Chloroplasts, like mitochondria, contain their own DNA, which is thought to be inherited from their ancestor—a photosynthetic cyanobacterium that was engulfed by an early eukaryotic cell. Chloroplasts cannot be made by the plant cell and must be inherited by each daughter cell during cell division.
With one exception (the amoeboid Paulinella chromatophora), all chloroplasts can probably be traced back to a single endosymbiotic event, when a cyanobacterium was engulfed by the eukaryote. Despite this, chloroplasts can be found in an extremely wide set of organisms, some not even directly related to each other—a consequence of many secondary and even tertiary endosymbiotic events.
The word chloroplast is derived from the Greek words chloros (χλωρός), which means green, and plastes (πλάστης), which means "the one who forms"
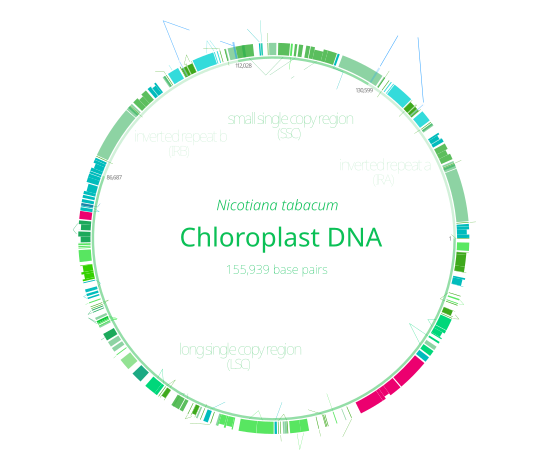
Chloroplast DNA
Chloroplasts have their own DNA, often abbreviated as ctDNA, or cpDNA. It is also known as the plastome. Its existence was first proved in 1962, and first sequenced in 1986—when two Japanese research teams sequenced the chloroplast DNA of liverwort and tobacco. Since then, hundreds of chloroplast DNAs from various species have been sequenced, but they're mostly those of land plants and green algae—glaucophytes, red algae, and other algal groups are extremely underrepresented, potentially introducing some bias in views of "typical" chloroplast DNA structure and content.
Molecular structure
replication origin regions
atp-dependent protease
ribosomal proteins
Chloroplast DNA Interactive gene map of chloroplast DNA from Nicotiana tabacum. Segments with labels on the inside reside on the B strand of DNA, segments with labels on the outside are on the A strand. Notches indicate introns.
With few exceptions, most chloroplasts have their entire chloroplast genome combined into a single large circular DNA molecule, typically 120,000–170,000 base pairs long. They can have a contour length of around 30–60 micrometers, and have a mass of about 80–130 million daltons.
While usually thought of as a circular molecule, there is some evidence that chloroplast DNA molecules more often take on a linear shape.
Inverted repeats
Many chloroplast DNAs contain two inverted repeats, which separate a long single copy section (LSC) from a short single copy section (SSC). While a given pair of inverted repeats are rarely completely identical, they are always very similar to each other, apparently resulting from concerted evolution.
The inverted repeats vary wildly in length, ranging from 4,000 to 25,000 base pairs long each and containing as few as four or as many as over 150 genes. Inverted repeats in plants tend to be at the upper end of this range, each being 20,000–25,000 base pairs long.
The inverted repeat regions are highly conserved among land plants, and accumulate few mutations. Similar inverted repeats exist in the genomes of cyanobacteria and the other two chloroplast lineages (glaucophyta and rhodophyceae), suggesting that they predate the chloroplast, though some chloroplast DNAs have since lost or flipped the inverted repeats (making them direct repeats). It is possible that the inverted repeats help stabilize the rest of the chloroplast genome, as chloroplast DNAs which have lost some of the inverted repeat segments tend to get rearranged more.
Nucleoids
New chloroplasts may contain up to 100 copies of their DNA, though the number of chloroplast DNA copies decreases to about 15–20 as the chloroplasts age. They are usually packed into nucleoids, which can contain several identical chloroplast DNA rings. Many nucleoids can be found in each chloroplast. In primitive red algae, the chloroplast DNA nucleoids are clustered in the center of the chloroplast, while in green plants and green algae, the nucleoids are dispersed throughout the stroma.
Though chloroplast DNA is not associated with true histones, in red algae, similar proteins that tightly pack each chloroplast DNA ring into a nucleoid have been found.
Structure
In land plants, chloroplasts are generally lens-shaped, 3–10 μm in diameter and 1–3 μm thick. Corn seedling chloroplasts are ≈20 µm3 in volume. Greater diversity in chloroplast shapes exists among the algae, which often contain a single chloroplast that can be shaped like a net (e.g., Oedogonium), a cup (e.g., Chlamydomonas), a ribbon-like spiral around the edges of the cell (e.g., Spirogyra), or slightly twisted bands at the cell edges (e.g., Sirogonium). Some algae have two chloroplasts in each cell; they are star-shaped in Zygnema, or may follow the shape of half the cell in order Desmidiales. In some algae, the chloroplast takes up most of the cell, with pockets for the nucleus and other organelles. for example, some species of Chlorella have a cup-shaped chloroplast that occupies much of the cell.
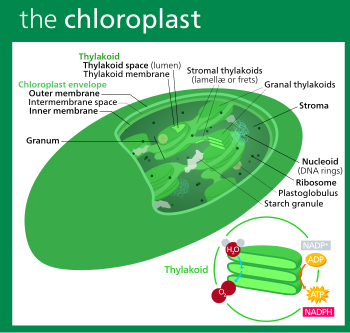
All chloroplasts have at least three membrane systems—the outer chloroplast membrane, the inner chloroplast membrane, and the thylakoid system. Chloroplasts that are the product of secondary endosymbiosis may have additional membranes surrounding these three. Inside the outer and inner chloroplast membranes is the chloroplast stroma, a semi-gel-like fluid that makes up much of a chloroplast's volume, and in which the thylakoid system floats.
There are some common misconceptions about the outer and inner chloroplast membranes. The fact that chloroplasts are surrounded by a double membrane is often cited as evidence that they are the descendants of endosymbiotic cyanobacteria. This is often interpreted as meaning the outer chloroplast membrane is the product of the host's cell membrane infolding to form a vesicle to surround the ancestral cyanobacterium—which is not true—both chloroplast membranes are homologous to the cyanobacterium's original double membranes.
The chloroplast double membrane is also often compared to the mitochondrial double membrane. This is not a valid comparison—the inner mitochondria membrane is used to run proton pumps and carry out oxidative phosphorylation across to generate ATP energy. The only chloroplast structure that can considered analogous to it is the internal thylakoid system. Even so, in terms of "in-out", the direction of chloroplast H+ ion flow is in the opposite direction compared to oxidative phosphorylation in mitochondria. In addition, in terms of function, the inner chloroplast membrane, which regulates metabolite passage and synthesizes some materials, has no counterpart in the mitochondrion.
Outer chloroplast membrane
The outer chloroplast membrane is a semi-porous membrane that small molecules and ions can easily diffuse across. However, it is not permeable to larger proteins, so chloroplast polypeptides being synthesized in the cell cytoplasm must be transported across the outer chloroplast membrane by the TOC complex, or translocon on the outer chloroplast membrane.
The chloroplast membranes sometimes protrude out into the cytoplasm, forming a stromule, or stroma-containing tubule. Stromules are very rare in chloroplasts, and are much more common in other plastids like chromoplasts and amyloplasts in petals and roots, respectively. They may exist to increase the chloroplast's surface area for cross-membrane transport, because they are often branched and tangled with the endoplasmic reticulum. When they were first observed in 1962, some plant biologists dismissed the structures as artifactual, claiming that stromules were just oddly shaped chloroplasts with constricted regions or dividing chloroplasts. However, there is a growing body of evidence that stromules are functional, integral features of plant cell plastids, not merely artifacts.
Intermembrane space and peptidoglycan wall
Usually, a thin intermembrane space about 10–20 nanometers thick exists between the outer and inner chloroplast membranes.
Glaucophyte algal chloroplasts have a peptidoglycan layer between the chloroplast membranes. It corresponds to the peptidoglycan cell wall of their cyanobacterial ancestors, which is located between their two cell membranes. These chloroplasts are called muroplasts (from Latin "mura", meaning "wall"). Other chloroplasts have lost the cyanobacterial wall, leaving an intermembrane space between the two chloroplast envelope membranes.
Inner chloroplast membrane
The inner chloroplast membrane borders the stroma and regulates passage of materials in and out of the chloroplast. After passing through the TOC complex in the outer chloroplast membrane, polypeptides must pass through the TIC complex (translocon on the inner chloroplast membrane) which is located in the inner chloroplast membrane.
In addition to regulating the passage of materials, the inner chloroplast membrane is where fatty acids, lipids, and carotenoids are synthesized.
Peripheral reticulum
Some chloroplasts contain a structure called the chloroplast peripheral reticulum. It is often found in the chloroplasts of C4 plants, though it has also been found in some C3angiosperms, and even some gymnosperms. The chloroplast peripheral reticulum consists of a maze of membranous tubes and vesicles continuous with the inner chloroplast membrane that extends into the internal stromal fluid of the chloroplast. Its purpose is thought to be to increase the chloroplast's surface area for cross-membrane transport between its stroma and the cell cytoplasm. The small vesicles sometimes observed may serve as transport vesicles to shuttle stuff between the thylakoids and intermembrane space.
Stroma
The protein-rich, alkaline, aqueous fluid within the inner chloroplast membrane and outside of the thylakoid space is called the stroma, which corresponds to the cytosol of the original cyanobacterium. Nucleoids of chloroplast DNA, chloroplast ribosomes, the thylakoid system with plastoglobuli, starch granules, and many proteins can be found floating around in it. The Calvin cycle, which fixes CO2 into sugar takes place in the stroma.
Chloroplast ribosomes
Chloroplasts have their own ribosomes, which they use to synthesize a small fraction of their proteins. Chloroplast ribosomes are about two-thirds the size of cytoplasmic ribosomes (around 17 nm vs 25 nm). They take mRNAs transcribed from the chloroplast DNA and translate them into protein. While similar to bacterial ribosomes, chloroplast translation is more complex than in bacteria, so chloroplast ribosomes include some chloroplast-unique features. Small subunit ribosomal RNAs in several Chlorophyta and euglenid chloroplasts lack motifs for shine-dalgarno sequence recognition, which is considered essential for translation initiation in most chloroplasts and prokaryotes. Such loss is also rarely observed in other plastids and prokaryotes.
Plastoglobuli
Plastoglobuli (singular plastoglobulus, sometimes spelled plastoglobule(s)), are spherical bubbles of lipids and proteinsabout 45–60 nanometers across. They are surrounded by a lipid monolayer. Plastoglobuli are found in all chloroplasts, but become more common when the chloroplast is under oxidative stress, or when it ages and transitions into a gerontoplast. Plastoglobuli also exhibit a greater size variation under these conditions. They are also common in etioplasts, but decrease in number as the etioplasts mature into chloroplasts.
Plastoglubuli contain both structural proteins and enzymes involved in lipid synthesis and metabolism. They contain many types of lipids including plastoquinone, vitamin E, carotenoids and chlorophylls.
Plastoglobuli were once thought to be free-floating in the stroma, but it is now thought that they are permanently attached either to a thylakoid or to another plastoglobulus attached to a thylakoid, a configuration that allows a plastoglobulus to exchange its contents with the thylakoid network. In normal green chloroplasts, the vast majority of plastoglobuli occur singularly, attached directly to their parent thylakoid. In old or stressed chloroplasts, plastoglobuli tend to occur in linked groups or chains, still always anchored to a thylakoid.
Plastoglobuli form when a bubble appears between the layers of the lipid bilayer of the thylakoid membrane, or bud from existing plastoglubuli—though they never detach and float off into the stroma. Practically all plastoglobuli form on or near the highly curved edges of the thylakoid disks or sheets. They are also more common on stromal thylakoids than on granal ones.
Starch granules
Starch granules are very common in chloroplasts, typically taking up 15% of the organelle's volume, though in some other plastids like amyloplasts, they can be big enough to distort the shape of the organelle. Starch granules are simply accumulations of starch in the stroma, and are not bounded by a membrane.
Starch granules appear and grow throughout the day, as the chloroplast synthesizes sugars, and are consumed at night to fuel respiration and continue sugar export into the phloem, though in mature chloroplasts, it is rare for a starch granule to be completely consumed or for a new granule to accumulate.
Starch granules vary in composition and location across different chloroplast lineages. In red algae, starch granules are found in the cytoplasm rather than in the chloroplast.In C4 plants, mesophyll chloroplasts, which do not synthesize sugars, lack starch granules.
Rubisco
The chloroplast stroma contains many proteins, though the most common and important is Rubisco, which is probably also the most abundant protein on the planet. Rubisco is the enzyme that fixes CO2 into sugar molecules. In C3 plants, rubisco is abundant in all chloroplasts, though in C4 plants, it is confined to the bundle sheath chloroplasts, where the Calvin cycle is carried out in C4 plants.
Pyrenoids
The chloroplasts of some hornworts and algae contain structures called pyrenoids. They are not found in higher plants. Pyrenoids are roughly spherical and highly refractive bodies which are a site of starch accumulation in plants that contain them. They consist of a matrix opaque to electrons, surrounded by two hemispherical starch plates. The starch is accumulated as the pyrenoids mature. In algae with carbon concentrating mechanisms, the enzyme rubisco is found in the pyrenoids. Starch can also accumulate around the pyrenoids when CO2 is scarce. Pyrenoids can divide to form new pyrenoids, or be produced "de novo".
Thylakoid system
Suspended within the chloroplast stroma is the thylakoid system, a highly dynamic collection of membranous sacks called thylakoids where chlorophyll is found and the light reactions of photosynthesis happen. In most vascular plant chloroplasts, the thylakoids are arranged in stacks called grana, though in certain C4 plant chloroplasts and some algal chloroplasts, the thylakoids are free floating.
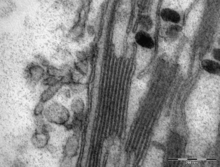
Granal structure
Using a light microscope, it is just barely possible to see tiny green granules—which were named grana. With electron microscopy, it became possible to see the thylakoid system in more detail, revealing it to consist of stacks of flat thylakoids which made up the grana, and long interconnecting stromal thylakoids which linked different grana. In the transmission electron microscope, thylakoid membranes appear as alternating light-and-dark bands, 8.5 nanometers thick.
For a long time, the three-dimensional structure of the thylakoid system has been unknown or disputed. One model has the granum as a stack of thylakoids linked by helical stromal thylakoids; the other has the granum as a single folded thylakoid connected in a "hub and spoke" way to other grana by stromal thylakoids. While the thylakoid system is still commonly depicted according to the folded thylakoid model, it was determined in 2011 that the stacked and helical thylakoids model is correct.
In the helical thylakoid model, grana consist of a stack of flattened circular granal thylakoids that resemble pancakes. Each granum can contain anywhere from two to a hundred thylakoids, though grana with 10–20 thylakoids are most common. Wrapped around the grana are helicoid stromal thylakoids, also known as frets or lamellar thylakoids. The helices ascend at an angle of 20–25°, connecting to each granal thylakoid at a bridge-like slit junction. The helicoids may extend as large sheets that link multiple grana, or narrow to tube-like bridges between grana.[111] While different parts of the thylakoid system contain different membrane proteins, the thylakoid membranes are continuous and the thylakoid space they enclose form a single continuous labyrinth.
Thylakoids
Thylakoids (sometimes spelled thylakoïds), are small interconnected sacks which contain the membranes that the light reactions of photosynthesis take place on. The word thylakoid comes from the Greek word thylakos which means "sack".
Embedded in the thylakoid membranes are important protein complexes which carry out the light reactions of photosynthesis. Photosystem II and photosystem I contain light-harvesting complexes with chlorophyll and carotenoids that absorb light energy and use it to energize electrons. Molecules in the thylakoid membrane use the energized electrons to pump hydrogen ions into the thylakoid space, decreasing the pH and turning it acidic. ATP synthase is a large protein complex that harnesses the concentration gradient of the hydrogen ions in the thylakoid space to generate ATP energy as the hydrogen ions flow back out into the stroma—much like a dam turbine.
There are two types of thylakoids—granal thylakoids, which are arranged in grana, and stromal thylakoids, which are in contact with the stroma. Granal thylakoids are pancake-shaped circular disks about 300–600 nanometers in diameter. Stromal thylakoids are helicoid sheets that spiral around grana. The flat tops and bottoms of granal thylakoids contain only the relatively flat photosystem II protein complex. This allows them to stack tightly, forming grana with many layers of tightly appressed membrane, called granal membrane, increasing stability and surface area for light capture.
In contrast, photosystem I and ATP synthase are large protein complexes which jut out into the stroma. They can't fit in the appressed granal membranes, and so are found in the stromal thylakoid membrane—the edges of the granal thylakoid disks and the stromal thylakoids. These large protein complexes may act as spacers between the sheets of stromal thylakoids.
The number of thylakoids and the total thylakoid area of a chloroplast is influenced by light exposure. Shaded chloroplasts contain larger and more grana with more thylakoid membrane area than chloroplasts exposed to bright light, which have smaller and fewer grana and less thylakoid area. Thylakoid extent can change within minutes of light exposure or removal.
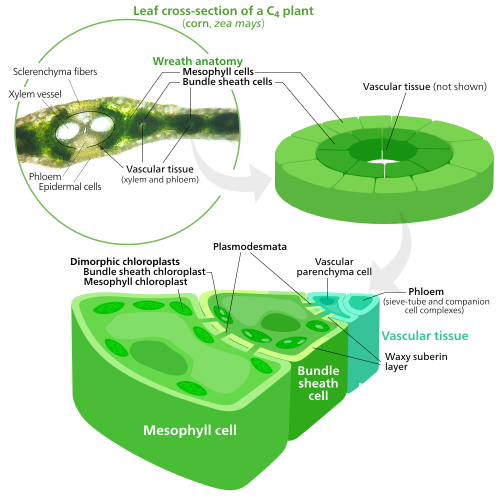
Specialized chloroplasts in C4 plants
C4 plants evolved a way to solve this—by spatially separating the light reactions and the Calvin cycle. The light reactions, which store light energy in ATP and NADPH, are done in the mesophyll cells of a C4 leaf. The Calvin cycle, which uses the stored energy to make sugar using rubisco, is done in the bundle sheath cells, a layer of cells surrounding a vein in a leaf.
As a result, chloroplasts in C4 mesophyll cells and bundle sheath cells are specialized for each stage of photosynthesis. In mesophyll cells, chloroplasts are specialized for the light reactions, so they lack rubisco, and have normal grana and thylakoids, which they use to make ATP and NADPH, as well as oxygen. They store CO2 in a four-carbon compound, which is why the process is called C4 photosynthesis. The four-carbon compound is then transported to the bundle sheath chloroplasts, where it drops off CO2 and returns to the mesophyll. Bundle sheath chloroplasts do not carry out the light reactions, preventing oxygen from building up in them and disrupting rubisco activity. Because of this, they lack thylakoids organized into grana stacks—though bundle sheath chloroplasts still have free-floating thylakoids in the stroma where they still carry out cyclic electron flow, a light-driven method of synthesizing ATP to power the Calvin cycle without generating oxygen. They lack photosystem II, and only have photosystem I—the only protein complex needed for cyclic electron flow. Because the job of bundle sheath chloroplasts is to carry out the Calvin cycle and make sugar, they often contain large starch grains.
Both types of chloroplast contain large amounts of chloroplast peripheral reticulum, which they use to get more surface area to transport stuff in and out of them.Mesophyll chloroplasts have a little more peripheral reticulum than bundle sheath chloroplasts.
Location
Distribution in a plant
Not all cells in a multicellular plant contain chloroplasts. All green parts of a plant contain chloroplasts—the chloroplasts, or more specifically, the chlorophyll in them are what make the photosynthetic parts of a plant green. The plant cells which contain chloroplasts are usually parenchyma cells, though chloroplasts can also be found in collenchyma tissue. A plant cell which contains chloroplasts is known as a chlorenchyma cell. A typical chlorenchyma cell of a land plant contains about 10 to 100 chloroplasts.
In some plants such as cacti, chloroplasts are found in the stems, though in most plants, chloroplasts are concentrated in the leaves. One square millimeter of leaf tissue can contain half a million chloroplasts. Within a leaf, chloroplasts are mainly found in the mesophyll layers of a leaf, and the guard cells of stomata. Palisade mesophyll cells can contain 30–70 chloroplasts per cell, while stomatal guard cells contain only around 8–15 per cell, as well as much less chlorophyll. Chloroplasts can also be found in the bundle sheath cells of a leaf, especially in C4 plants, which carry out the Calvin cycle in their bundle sheath cells. They are often absent from the epidermis of a leaf.
Cellular location
Chloroplast movement
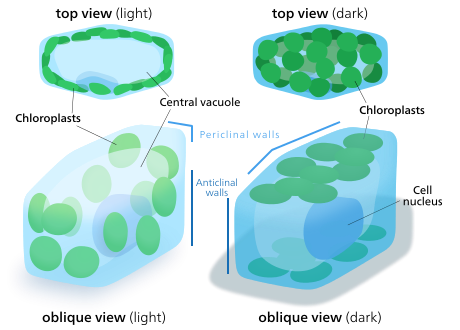
The chloroplasts of plant and algal cells can orient themselves to best suit the available light. In low-light conditions, they will spread out in a sheet—maximizing the surface area to absorb light. Under intense light, they will seek shelter by aligning in vertical columns along the plant cell's cell wall or turning sideways so that light strikes them edge-on. This reduces exposure and protects them from photooxidative damage.This ability to distribute chloroplasts so that they can take shelter behind each other or spread out may be the reason why land plants evolved to have many small chloroplasts instead of a few big ones. Chloroplast movement is considered one of the most closely regulated stimulus-response systems that can be found in plants. Mitochondria have also been observed to follow chloroplasts as they move.
In higher plants, chloroplast movement is run by phototropins, blue light photoreceptors also responsible for plant phototropism. In some algae, mosses, ferns, and flowering plants, chloroplast movement is influenced by red light in addition to blue light, though very long red wavelengths inhibit movement rather than speeding it up. Blue light generally causes chloroplasts to seek shelter, while red light draws them out to maximize light absorption.
Studies of Vallisneria gigantea, an aquatic flowering plant, have shown that chloroplasts can get moving within five minutes of light exposure, though they don't initially show any net directionality. They may move along microfilament tracks, and the fact that the microfilament mesh changes shape to form a honeycomb structure surrounding the chloroplasts after they have moved suggests that microfilaments may help to anchor chloroplasts in place.
Function and chemistry
Guard cell chloroplasts
Plant innate immunity
Unlike most epidermal cells, the guard cells of plant stomata contain relatively well-developed chloroplasts. However, exactly what they do is controversial.
Plants lack specialized immune cells—all plant cells participate in the plant immune response. Chloroplasts, along with the nucleus, cell membrane, and endoplasmic reticulum, are key players in pathogen defense. Due to its role in a plant cell's immune response, pathogens frequently target the chloroplast.
Plants have two main immune responses—the hypersensitive response, in which infected cells seal themselves off and undergo programmed cell death, and systemic acquired resistance, where infected cells release signals warning the rest of the plant of a pathogen's presence. Chloroplasts stimulate both responses by purposely damaging their photosynthetic system, producing reactive oxygen species. High levels of reactive oxygen species will cause the hypersensitive response. The reactive oxygen species also directly kill any pathogens within the cell. Lower levels of reactive oxygen species initiate systemic acquired resistance, triggering defense-molecule production in the rest of the plant.
In some plants, chloroplasts are known to move closer to the infection site and the nucleus during an infection.
Chloroplasts can serve as cellular sensors. After detecting stress in a cell, which might be due to a pathogen, chloroplasts begin producing molecules like salicylic acid, jasmonic acid, nitric oxide and reactive oxygen species which can serve as defense-signals. As cellular signals, reactive oxygen species are unstable molecules, so they probably don't leave the chloroplast, but instead pass on their signal to an unknown second messenger molecule. All these molecules initiate retrograde signaling—signals from the chloroplast that regulate gene expression in the nucleus.
In addition to defense signaling, chloroplasts, with the help of the peroxisomes, help synthesize an important defense molecule, jasmonate. Chloroplasts synthesize all the fatty acids in a plant celllinoleic acid, a fatty acid, is a precursor to jasmonate.
Photosynthesis
One of the main functions of the chloroplast is its role in photosynthesis, the process by which light is transformed into chemical energy, to subsequently produce food in the form of sugars. Water (H2O) and carbon dioxide (CO2) are used in photosynthesis, and sugar and oxygen (O2) is made, using light energy. Photosynthesis is divided into two stages—the light reactions, where water is split to produce oxygen, and the dark reactions, or Calvin cycle, which builds sugar molecules from carbon dioxide. The two phases are linked by the energy carriers adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADP+).
Light reactions
The light reactions take place on the thylakoid membranes. They take light energy and store it in NADPH, a form of NADP+, and ATP to fuel the dark reactions.
Energy carriers
ATP is the phosphorylated version of adenosine diphosphate (ADP), which stores energy in a cell and powers most cellular activities. ATP is the energized form, while ADP is the (partially) depleted form. NADP+ is an electron carrier which ferries high energy electrons. In the light reactions, it gets reduced, meaning it picks up electrons, becoming NADPH.
Photophosphorylation
Like mitochondria, chloroplasts use the potential energy stored in an H+, or hydrogen ion gradient to generate ATP energy. The two photosystems capture light energy to energize electrons taken from water, and release them down an electron transport chain. The molecules between the photosystems harness the electrons' energy to pump hydrogen ions into the thylakoid space, creating a concentration gradient, with more hydrogen ions (up to a thousand times as many) inside the thylakoid system than in the stroma. The hydrogen ions in the thylakoid space then diffuse back down their concentration gradient, flowing back out into the stroma through ATP synthase. ATP synthase uses the energy from the flowing hydrogen ions to phosphorylate adenosine diphosphate into adenosine triphosphate, or ATP. Because chloroplast ATP synthase projects out into the stroma, the ATP is synthesized there, in position to be used in the dark reactions.
NADP+ reduction
Electrons are often removed from the electron transport chains to charge NADP+ with electrons, reducing it to NADPH. Like ATP synthase, ferredoxin-NADP+ reductase, the enzyme that reduces NADP+, releases the NADPH it makes into the stroma, right where it is needed for the dark reactions.
Because NADP+ reduction removes electrons from the electron transport chains, they must be replaced—the job of photosystem II, which splits water molecules (H2O) to obtain the electrons from its hydrogen atoms.
Cyclic photophosphorylation
While photosystem II photolyzes water to obtain and energize new electrons, photosystem I simply reenergizes depleted electrons at the end of an electron transport chain. Normally, the reenergized electrons are taken by NADP+, though sometimes they can flow back down more H+-pumping electron transport chains to transport more hydrogen ions into the thylakoid space to generate more ATP. This is termed cyclic photophosphorylation because the electrons are recycled. Cyclic photophosphorylation is common in C4plants, which need more ATP than NADPH.
Dark reactions
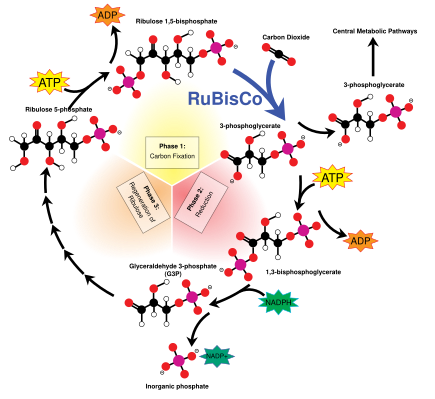
The Calvin cycle, also known as the dark reactions, is a series of biochemical reactions that fixes CO2 into G3P sugar molecules and uses the energy and electrons from the ATP and NADPH made in the light reactions. The Calvin cycle takes place in the stroma of the chloroplast.
While named "the dark reactions", in most plants, they take place in the light, since the dark reactions are dependent on the products of the light reactions.
Carbon fixation and G3P synthesis
The Calvin cycle starts by using the enzyme Rubisco to fix CO2 into five-carbon Ribulose bisphosphate (RuBP) molecules. The result is unstable six-carbon molecules that immediately break down into three-carbon molecules called 3-phosphoglyceric acid, or 3-PGA. The ATP and NADPH made in the light reactions is used to convert the 3-PGA into glyceraldehyde-3-phosphate, or G3P sugar molecules. Most of the G3P molecules are recycled back into RuBP using energy from more ATP, but one out of every six produced leaves the cycle—the end product of the dark reactions.
Sugars and starches
Glyceraldehyde-3-phosphate can double up to form larger sugar molecules like glucose and fructose. These molecules are processed, and from them, the still larger sucrose, a disaccharide commonly known as table sugar, is made, though this process takes place outside of the chloroplast, in the cytoplasm.
Alternatively, glucose monomers in the chloroplast can be linked together to make starch, which accumulates into the starch grains found in the chloroplast. Under conditions such as high atmospheric CO2concentrations, these starch grains may grow very large, distorting the grana and thylakoids. The starch granules displace the thylakoids, but leave them intact. Waterlogged roots can also cause starch buildup in the chloroplasts, possibly due to less sucrose being exported out of the chloroplast (or more accurately, the plant cell). This depletes a plant's free phosphate supply, which indirectly stimulates chloroplast starch synthesis. While linked to low photosynthesis rates, the starch grains themselves may not necessarily interfere significantly with the efficiency of photosynthesis, and might simply be a side effect of another photosynthesis-depressing factor.
Photorespiration
Photorespiration can occur when the oxygen concentration is too high. Rubisco cannot distinguish between oxygen and carbon dioxide very well, so it can accidentally add O2 instead of CO2 to RuBP. This process reduces the efficiency of photosynthesis—it consumes ATP and oxygen, releases CO2, and produces no sugar. It can waste up to half the carbon fixed by the Calvin cycle. Several mechanisms have evolved in different lineages that raise the carbon dioxide concentration relative to oxygen within the chloroplast, increasing the efficiency of photosynthesis. These mechanisms are called carbon dioxide concentrating mechanisms, or CCMs. These include Crassulacean acid metabolism, C4 carbon fixation, and pyrenoids. Chloroplasts in C4 plants are notable as they exhibit a distinct chloroplast dimorphism.
pH
Because of the H+ gradient across the thylakoid membrane, the interior of the thylakoid is acidic, with a pH around 4, while the stroma is slightly basic, with a pH of around 8. The optimal stroma pH for the Calvin cycle is 8.1, with the reaction nearly stopping when the pH falls below 7.3.
CO2 in water can form carbonic acid, which can disturb the pH of isolated chloroplasts, interfering with photosynthesis, even though CO2 is used in photosynthesis. However, chloroplasts in living plant cells are not affected by this as much.
Chloroplasts can pump K+ and H+ ions in and out of themselves using a poorly understood light-driven transport system.
In the presence of light, the pH of the thylakoid lumen can drop up to 1.5 pH units, while the pH of the stroma can rise by nearly one pH unit.
Amino acid synthesis
Chloroplasts alone make almost all of a plant cell's amino acids in their stroma except the sulfur-containing ones like cysteine and methionine. Cysteine is made in the chloroplast (the proplastid too) but it is also synthesized in the cytosol and mitochondria, probably because it has trouble crossing membranes to get to where it is needed.The chloroplast is known to make the precursors to methionine but it is unclear whether the organelle carries out the last leg of the pathway or if it happens in the cytosol.
Other nitrogen compounds
Chloroplasts make all of a cell's purines and pyrimidines—the nitrogenous bases found in DNA and RNA. They also convert nitrite (NO2−) into ammonia (NH3) which supplies the plant with nitrogen to make its amino acids and nucleotides.
References
1. Wikipedia free encyclopedia.
2. CELL BIOLOGY by Dr CB POWER
- Publisher: Himalaya Publishing House (2010)
- Language: English
- ISBN-10: 9350246694
- ISBN-13: 978-9350246696
- ASIN: B003WWDSPC













No comments:
Post a Comment